Implantable cardioverterdefibrillator
Definition
The implantable cardioverter-defibrillator (ICD) is a surgically implanted electronic device that directs an electric charge directly into the heart to treat life-threatening arrhythmias.
Purpose
The implantable cardioverter-defibrillator is used to detect and stop life-threatening arrhythmias and restore a productive heartbeat that is able to provide adequate cardiac output to sustain life. The exact indications for the implantation of the device are controversial, but patients suffering from ventricular fibrillation (unproductive heartbeat), ventricular tachycardia (abnormally fast heartbeat), long QT syndrome (an inherited heart disease), or others at risk for sudden cardiac death are potential candidates for this device. A study by the National Institute for Heart, Lung, and Blood of the National Institutes of Health showed a significant increase in survival for patients suffering from ventricular arrhythmias when ICD implant is compared to medication. Several follow-up studies indicate that this may be due to the marked increase in survival for the sickest patients, generally defined as those having a heart weakened to less than 50% of normal, as measured by the ability of the left side of the heart to pump blood. Overall, studies have documented a very low mortality rate of 1–2% annually
Demographics
ICD implant is limited to patients that face the risk of sudden cardiac death from sustained ventricular arrhythmia, including ventricular tachycardia and ventricular fibrillation. Less than 1% of the more than 100,000 device implants done in the United States are performed on pediatric patients. Reduction in the risk of sudden cardiac death improves to less than 2% for both populations.
Diagnosis
Patients experiencing syncope (fainting) will be monitored with a cardiac monitor for arrhythmias. Following unsuccessful medical treatment for sustained ventricular arrhythmias, ICD implant will be indicated.
Description
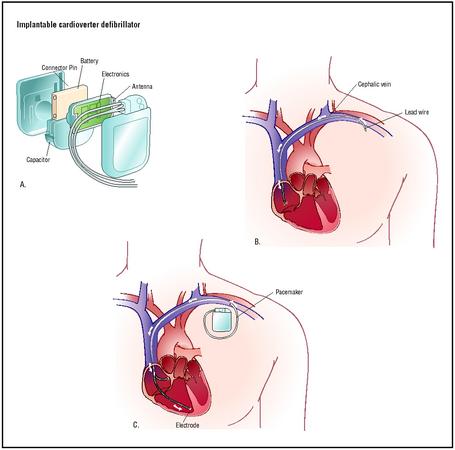
Similar in structure to a pacemaker, an ICD has three main components: a generator, leads, and an electrode. The generator is encased in a small rectangular container, usually about 2 in (5 cm) wide and around 3 oz (85 g) in weight. Even smaller generators have been developed, measuring 1 in (2.5 cm) in diameter and weighing about 0.5 oz (14 g). The generator is powered by lithium batteries and is responsible for generating the electric shock. The generator is controlled by a computer chip that can be programmed to follow specific steps according to the input gathered from the heart. The programming is initially set and can be changed using a wand programmer, a device that communicates by radio waves through the chest of the patient after implantation. One or two leads, or wires, are attached to the generator. These wires are generally made of platinum with an insulating coating of either silicone or polyurethane. The leads carry the electric shock from the generator. At the tip of each lead is a tiny device called an electrode that delivers the necessary electrical shock to the heart. Thus, the electric shock is created by the generator, carried by the leads, and delivered by the electrodes to the heart. The decision of where to put the leads depends on the needs of the patient, but they can be located in the left ventricle, the left atrium, or both.
According to the American College of Cardiology, more than 100,000 persons worldwide currently have an ICD. The battery-powered device rescues the patient from a life-threatening arrhythmia by performing a number of functions in order to reestablish normal heart rhythm, which varies with the particular problem of the patient. Specifically, if encountered with ventricular tachycardia, many devices will begin treatment with a pacing regimen. If the tachycardia is not too fast, the ICD can deliver several pacing signals in a row. When those signals stop, the heart may go back to a normal rhythm. If the pacing treatment is not successful, many devices will move onto cardioversion . With cardioversion, a mild shock is sent to the heart to stop the fast heartbeat. If the problem detected is ventricular fibrillation, a stronger shock called a defibrillation is sent. This stronger shock can stop the fast rhythm and help the heartbeat return to normal. Finally, many ICDs can also detect heartbeats that are too slow; they can act like a pacemaker and bring the heart rate up to normal. ICDs that defibrillate both the ventricles and the atria have also been developed. Such devices not only provide dual-chamber pacing but also can distinguish ventricular from atrial fibrillation. Patients that experience both atrial and ventricle fibrillations, or atrial fibrillation alone, that would not be controlled with a single-chamber device are candidates for this kind of ICD.
Operation
ICD insertion is considered minor surgery, and can be performed in either an operating room or an electrophysiology laboratory. The insertion site in the chest will be cleaned, shaved, and numbed with local anesthetic. Generally, left-handed persons have ICDs implanted on the right side, and visa versa, to speed return to normal activities. Two small cuts (incisions) are made, one in the chest wall and one in a vein just under the collarbone. The wires of the ICD are passed through the vein and attached to the inner surface of the heart. The other ends of the wires are connected to the main box of the ICD, which is inserted into the tissue under the collarbone and above the breast. Once the ICD is implanted, the physician will test it several times before the anesthesia wears off by causing the heart to fibrillate and making sure the ICD responds properly. The doctor then closes the incision with sutures (stitches), staples, or surgical glue. The entire procedure takes about an hour.
Immediately following the procedure, a chest x ray will be taken to confirm the proper placement of the wires in the heart. The ICD's programming may be adjusted by passing the programming wand over the chest. After the initial operation, the physician may induce ventricular fibrillation or ventricular tachycardia one more time prior to the patient's discharge, although recent studies suggest that this final test is not generally necessary.
A short stay in the hospital is usually required following ICD insertion, but this varies with the patient's age and condition. If there are no complications, complete recovery from the procedure will take about four weeks. During that time, the wires will firmly take hold where they were placed. In the meantime, the patient should avoid heavy lifting or vigorous movements of the arm on the side of the ICD, or else the wires may become dislodged.
After implantation, the cardioverter-defibrillator is programmed to respond to rhythms above the patient's exercise heart rate. Once the device is in place, many tests will be conducted to ensure that the device is sensing and defibrillating properly. About 50% of patients with ICDs require a combination of drug therapy and the ICD.
Morbidity and mortality rates
Perioperative mortality demonstrates a 0.4–1.8% risk of death for primary non-thoracotomy implants. The ICD showed improved survival compared to medical therapy, improving by 38% at one year. There is a 96% survival rate at four years for those implanted with ICD. Less then 2% of patients require termination of the device, with a return to only medical therapy.
Normal results
Ventricular tachycardia can be successfully relieved by pacing in 96% of instances with the addition of defibrillation converting 98% of patients to a productive rhythm that is able to sustain cardiac output. Ventricular fibrillation is successfully converted in 98.6–98.8% of all cases. Atrial fibrillation and rapid ventricular response leads to erroneous fibrillation in as many as 11% of patients.
Risks
Environmental conditions that can affect the functioning of the ICD after installation include:
- strong electromagnetic fields such as those used in arcwelding
- contact sports
- shooting a rifle from the shoulder nearest the installation site
- cell phones used on that side of the body
- magnetic mattress pads such as those believed to treat arthritis
- some medical tests such as magnetic resonance imaging (MRI)
Environmental conditions often erroneously thought to affect ICDs include:
- microwave ovens (the waves only affect old, unshielded pacemakers and do not affect ICDs)
- airport security (although metal detector alarms could be set off, so patients should carry a card stating they have an ICD implanted)
- anti-theft devices in stores (although patients should avoid standing near the devices for prolonged periods)
Patients should also be instructed to memorize the manufacturer and make of their ICD. Although manufacturing defects and recalls are rare, they do occur and a patient should be prepared for that possibility.
Aftercare
In general, if the condition of the patient's heart, drug intake, and metabolic condition remain the same, the ICD requires only periodic checking every two months or so for battery strength and function. This is done by placing a special device over the ICD that allows signals to be sent over the telephone to the doctor, a process called trans-telephonic monitoring.
If changes in medications or physical condition occur, the doctor can adjust the ICD settings using a programmer, which involves placing the wand above the pacemaker and remotely changing the internal settings. One relatively common problem is the so-called "ICD storm," in which the machine inappropriately interprets an arrhythmia and gives a series of shocks. Reprogramming can sometimes help alleviate that problem.
When the periodic testing indicates that the battery is getting low, an elective ICD replacement operation is scheduled. The entire signal generator is replaced because the batteries are sealed within the case. The leads can often be left in place and reattached to the new generator. Batteries usually last from four to eight years.
Alternatives
Patients are treated with medical therapy to reduce the chance of arrhythmia. This alternative has been shown to have a higher rate of sudden death when compared to ICD over the initial three years of treatment, but has not been compared at five years. If the site of ventricular tachycardia generation can be mapped by electrophysiology studies, the aberrant cells can be removed or destroyed. Less then 5% of patients suffer peri-operative mortality with this cell removal.
Resources
books
Gersh, Bernard J., ed. Mayo Clinic Heart Book. New York: William Morrow and Company, Inc., 2000.
periodicals
Gregoratos, Gabriel, et al. "ACC/AHA Guidelines for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices." Journal of the American College of Cardiologists 31, no. 5 (April 1998): 1175–1209.
Moss, A. "Implantable Cardioverter-Defibrillator Therapy: The Sickest Patients Benefit Most." Circulation 101 (April 2000): 1638–1640.
Sears, Samuel F. Jr., et al. "Fear of Exertion Following ICD Storm: Considering ICD Shock and Learning History." Journal of Cardiopulmonary Rehabilitation 21 (January/February 2001): 47.
organizations
American Heart Association. National Center. 7272 Greenville Avenue, Dallas, TX, 75231-4596. (214) 373-6300. http://www.americanheart.org .
North American Society of Pacing and Electrophysiology. 6 Strathmore Road, Natick, MA, 01760-2499. (508) 647-0100. http://www.naspe.org/index.html .
other
"Implantable Cardioverter-Defibrillator." American Academy of Family Physicians. May 7, 2001. http://www.familydoctor.org/handouts/270.html .
"Implantable Cardioverter-Defibrillators (ICDs)" North American Society of Pacing and Electrophysiology. 2000. http://www.naspe.org/your_heart/treatments/icds.html .
Michelle L. Johnson, MS,JD
Allison J. Spiwak, MSBME
WHO PERFORMS THE PROCEDURE AND WHERE IS IT PERFORMED?
Electrophysiologists are specially trained cardiologists or thoracic surgeons who study and treat problems with the heart conduction system. In a hospital operating room, they often implant the ICD system and oversee the programming or reprogramming of the device. Electrophysiologists receive special continuing medical education to provide successful implantation. Implantation, follow-up, and replacement can be limited at any one institution, therefore an experienced well-trained electrophysiologist should perform these procedures.
QUESTIONS TO ASK THE DOCTOR
- How many of these procedures have been performed by the physician?
- What type of longevity can be expected from the device?
- What will happen during device activation?
- What precautions should be taken in the weeks immediately following implant?
- After implantation how long will it be before normal daily activities can be resumed such as driving, exercise, and work?
- What indications of device malfunction will there be, and when should emergency treatment be sought?
- What precautions should be taken by bystanders when the device activates?
- How will device recalls be communicated?
- Can psychological counseling benefit patient satisfaction and comfort?
My father has just been told he's dying , he has c.o.p.d . He had his defibrillator turned off today and we are all in pieces . He still has is pace maker but I know this will not interfere with a peaceful death whereas the defibrillator could cause an agonising one so not not much choice in reaching the sad decision to deactivate. The docter s have informed us he could go at any time or could be here till Christmas , just about two months . We have our lovely Dad at home and will continue to nurse him , he is also on oral morphine as and when required. Bit of a question ,but are there any studies on how long you can last once deactivation takes place ? Ive searched online to try to see how much longer my lovely Dad will be with me but cannot find anything apart from advice on when to deactivate and how .
Many thanks if you feel you may be able to answer my question . Lorraine.